Recent Study in Science Advances Reports Discovery of Ribosome-Associated Vesicles, a Novel Form of Endoplasmic Reticulum
Scientists representing an international consortium of labs—including postdoctoral associate Despoina Aslanoglou, PhD, Kenneth Fish, PhD, and the study’s senior author, Zach Freyberg, MD, PhD, from Pitt Psychiatry—has published a new study in Science Advances, reporting new insights into the organization of the endoplasmic reticulum.
The endoplasmic reticulum is a membrane network within a cell that is important in the synthesis and transport of proteins. “We wanted to better understand the organization of the endoplasmic reticulum because it controls protein synthesis. Understanding where new proteins are made is the first step in shedding new light into how they are made.” said Dr. Freyberg.
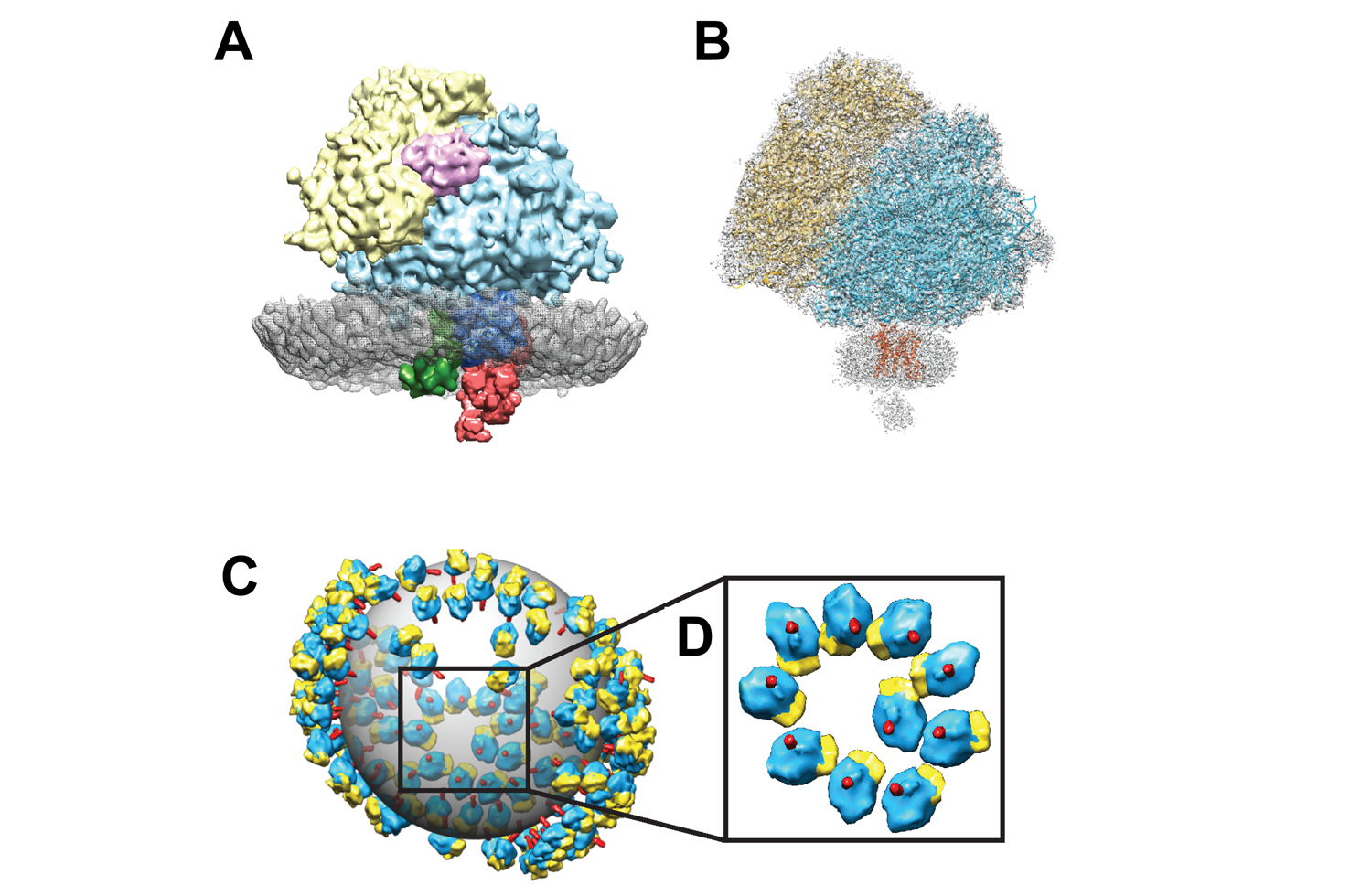
The team employed live-cell imaging to examine endoplasmic reticulum network dynamics with other intracellular organelles in real time. Using these methods, the team discovered ribosome-associated vesicles, novel endoplasmic reticulum-derived compartments that exist as discrete entities coexisting separately within cells from the full, intact endoplasmic reticulum architecture. They then followed these live-cell findings with a new form of imaging called in situ cryo-electron tomography. This allowed the team to visualize the ribosome-associated vesicles at molecular level resolution for the first time. Moreover, they discovered that these ribosome-associated vesicles are nearly universally found in different cell types and different species, including in people. Ultimately, the combination of these different imaging methods led the team to conclude that what they have been able to see represents an entirely new form of endoplasmic reticulum – adding a new organelle to our language of describing cells.
Although it remains uncertain what conditions or processes within the cell are associated with the emergence of ribosome-associated vesicles from the endoplasmic reticulum, it is possible that they represent a new mechanism by which secretory cells keep up with protein synthesis. The mobility of the ribosome-associate vesicles and their highly dynamic nature enables these structures to rapidly be transported to sites of local activity.
“Ultimately, cells in the body, including the brain, need to adapt to changes in the environment, and these changes underlie our resilience as human beings—to change behaviors, to lay down memories, to fight illness, and altering protein synthesis is at the center of all these processes,” said Dr. Freyberg. “However, the machinery behind these changes in protein synthesis remains very poorly understood. This discovery of a new, dynamic form of endoplasmic reticulum, capable of moving to sites of activity, may be a key piece of this long sought-after machinery and offers tremendous possibilities for unraveling many of the body’s mysteries both in health and disease. Stay tuned.”
Ribosome-associated vesicles: A dynamic subcompartment of the endoplasmic reticulum in secretory cells
Carter SD, Hampton CM, Langlois R, Melero R, Farino ZJ, Calderon MJ, Li W, Wallace CT, HT Ngoc, Grassucci RA, Siegmund SE, Pemberton J, Morgenstern TJ, Eisenman L, Aguilar JI, Greenberg NL, Levy ES, Yi E, Mitchell WG, Rice WJ, Wigge C, Pilli J, George EW, Aslanoglou D, Courel M, Freyberg RJ, Javitch JA, Wills ZP, Area-Gomez E, Shiva S, Bartolini F, Volchuk A, Murray SA, Aridor M, Fish KN, Walter P, Balla T, Fass D, Wolf SG, Watkins SC, Carazo JM, Jensen GJ, Frank J, Freyberg Z.
Science Advances. 01 Apr 2020: Vol. 6, no. 14, DOI: 10.1126/sciadv.aay9572
