American Journal of Psychiatry: New Research on Synaptic Variability and Cortical Gamma Oscillation Power in the Prefrontal Cortex in Schizophrenia
Impairments in certain cognitive processes, such as working memory, are core clinical features of schizophrenia. Working memory is associated with synchronized neural oscillatory activity at gamma band frequency in the prefrontal cortex, and the power of these oscillations during the performance of cognitive tasks is lower in individuals with schizophrenia. Thus, alterations in prefrontal cortex neural circuitry are thought to contribute to impaired gamma oscillations and working memory performance in schizophrenia.
In a recent study published in the American Journal of Psychiatry, Pitt Psychiatry scientists Wonjae Chung, MD, PhD (PGY4 resident, Psychiatry Research Pathway), Matthew Geramita, MD, PhD (PGY4 resident, Psychiatry Research Pathway), and David Lewis MD (Distinguished Professor of Psychiatry and Neuroscience, and Thomas Detre Professor of Academic Psychiatry), investigated whether variability in the strength of excitatory synaptic inputs to fast-spiking parvalbumin interneurons could contribute to lower prefrontal gamma power in schizophrenia.
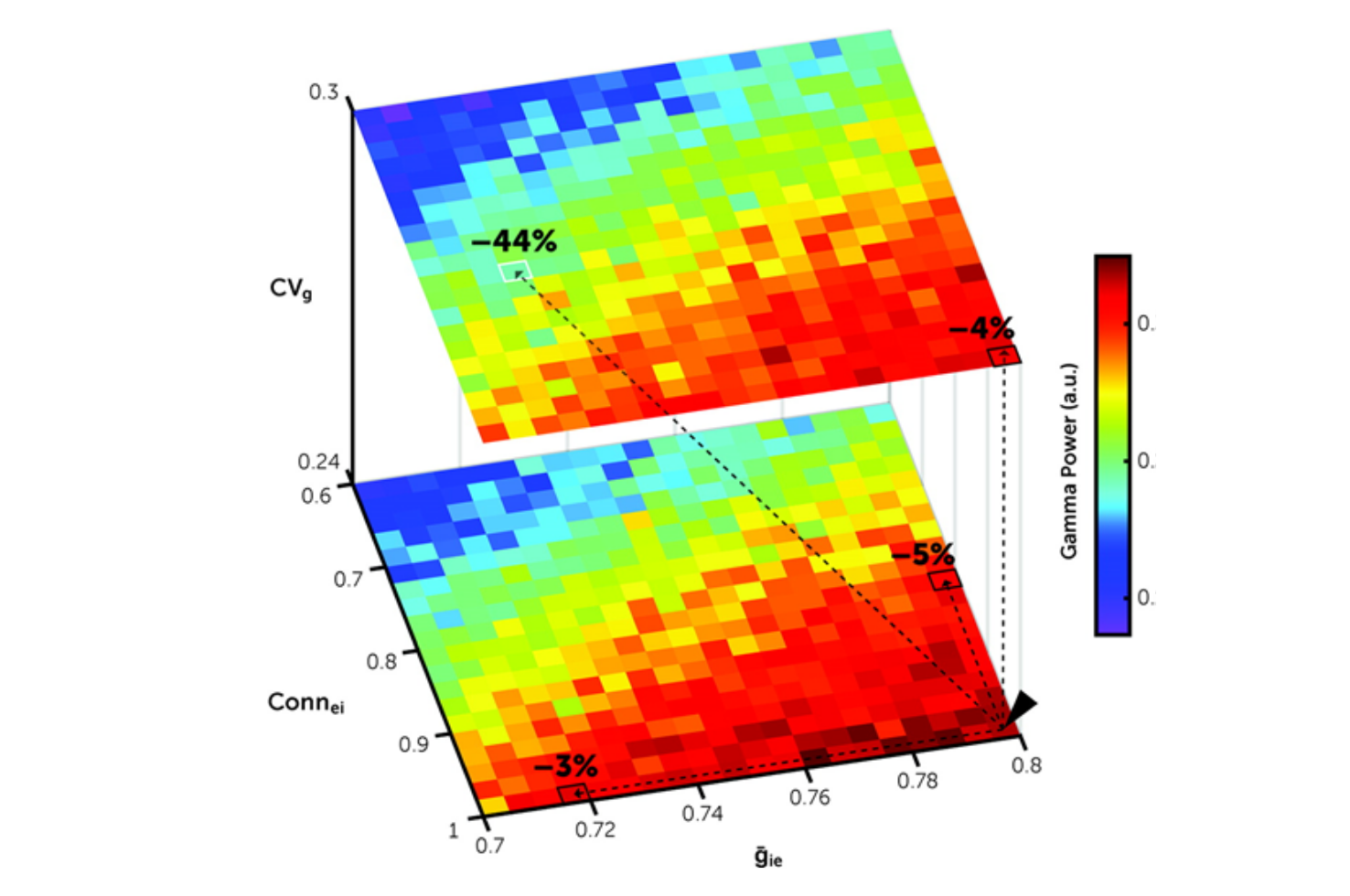
In postmortem prefrontal cortex samples from 20 matched pairs of schizophrenia and comparison subjects, the investigators quantified levels of vesicular glutamate transporter 1 and postsynaptic density 95 proteins to assess variability in the strength of excitatory synaptic inputs to parvalbumin interneurons. They then used a computational model network to simulate how variability in excitatory synaptic strength across fast-spiking interneurons regulates gamma power.
Findings from the study showed that the variability of vesicular glutamate transporter 1 and postsynaptic density 95 levels at excitatory inputs across parvalbumin interneurons was larger in schizophrenia relative to comparison subjects. This alteration was not influenced by schizophrenia-associated comorbid factors nor was it present in calretinin interneurons. In the model network, variability in excitatory synaptic strength across fast-spiking interneurons regulated gamma power by affecting network synchrony. Additionally, greater synaptic variability interacted synergistically with other synaptic alterations in schizophrenia (i.e., fewer excitatory inputs to fast-spiking interneurons and lower inhibitory strength from fast-spiking interneurons) to reduce gamma power. The findings suggest that greater variability in excitatory synaptic strength across parvalbumin interneurons, in combination with other modest synaptic alterations in these neurons, can markedly lower prefrontal cortex gamma power in schizophrenia.
“The neurobiology of schizophrenia has been conventionally studied by assessing the mean values of isolated synaptic measures. In this study, we combined a postmortem human brain study and computational modeling to demonstrate that schizophrenia is associated with alterations in the variability of synaptic strength, and that this alteration can interact synergistically with other modest synaptic alterations to produce marked deficits in prefrontal cortical gamma power. Thus, our study reveals synaptic variability as an important element of the disease process and suggests that prefrontal cortical dysfunction in schizophrenia may emerge from the dynamic interaction of multiple synaptic alterations,” said corresponding author, Dr. Chung.
“The findings of this study are both novel and important, and a great reflection on the effective collaboration of Wonjae and Matt, two of the already accomplished physician-scientists in our residency program,” said Dr. Lewis.
Synaptic Variability and Cortical Gamma Oscillation Power in Schizophrenia
Chung DW, Geramita MA, Lewis DA
American Journal of Psychiatry 2022; 179:277-287 https://doi.org/10.1176/appi.ajp.2021.21080798
