Translational Psychiatry: New Research on Dopaminergic Modulation of Pancreatic Hormone Secretion
Antipsychotic drugs can cause metabolic dysfunction that increases the risk for developing type 2 diabetes. These medications block dopamine D2-like receptors, including D2 and D3 receptors, which are expressed in pancreatic β-cells (in addition to the central nervous system). Dopamine and norepinephrine are catecholamines that act in the pancreas as peripheral regulators of metabolism. Pancreatic catecholamine signaling has also been increasingly implicated as a mechanism responsible for the metabolic disturbances produced by antipsychotic drugs.
In Translational Psychiatry, investigators including Despoina Aslanoglou, PhD (postdoctoral scholar) and Zachary Freyberg, MD, PhD (Assistant Professor of Psychiatry and Cell Biology) examined the mechanisms by which catecholamines modulate pancreatic hormone release.
“We wanted to understand how antipsychotic drugs, which target dopamine system in the brain, cause metabolic side effects in patients that receive these medications,” said Dr. Aslanoglou, the study’s first author. “Our work could set the basis for defining the theory of dopamine in metabolic dysfunction, and our findings can be useful for future drug design for both psychiatric and metabolic disorders.”
To evaluate whether human and mouse α-cells and β-cells express the machinery of catecholamine biosynthesis and catabolism, the team analyzed an RNA-sequencing data set from human α-cells and β-cells, alongside a comparable available RNA- sequencing data set from mouse α-cells and β-cells.
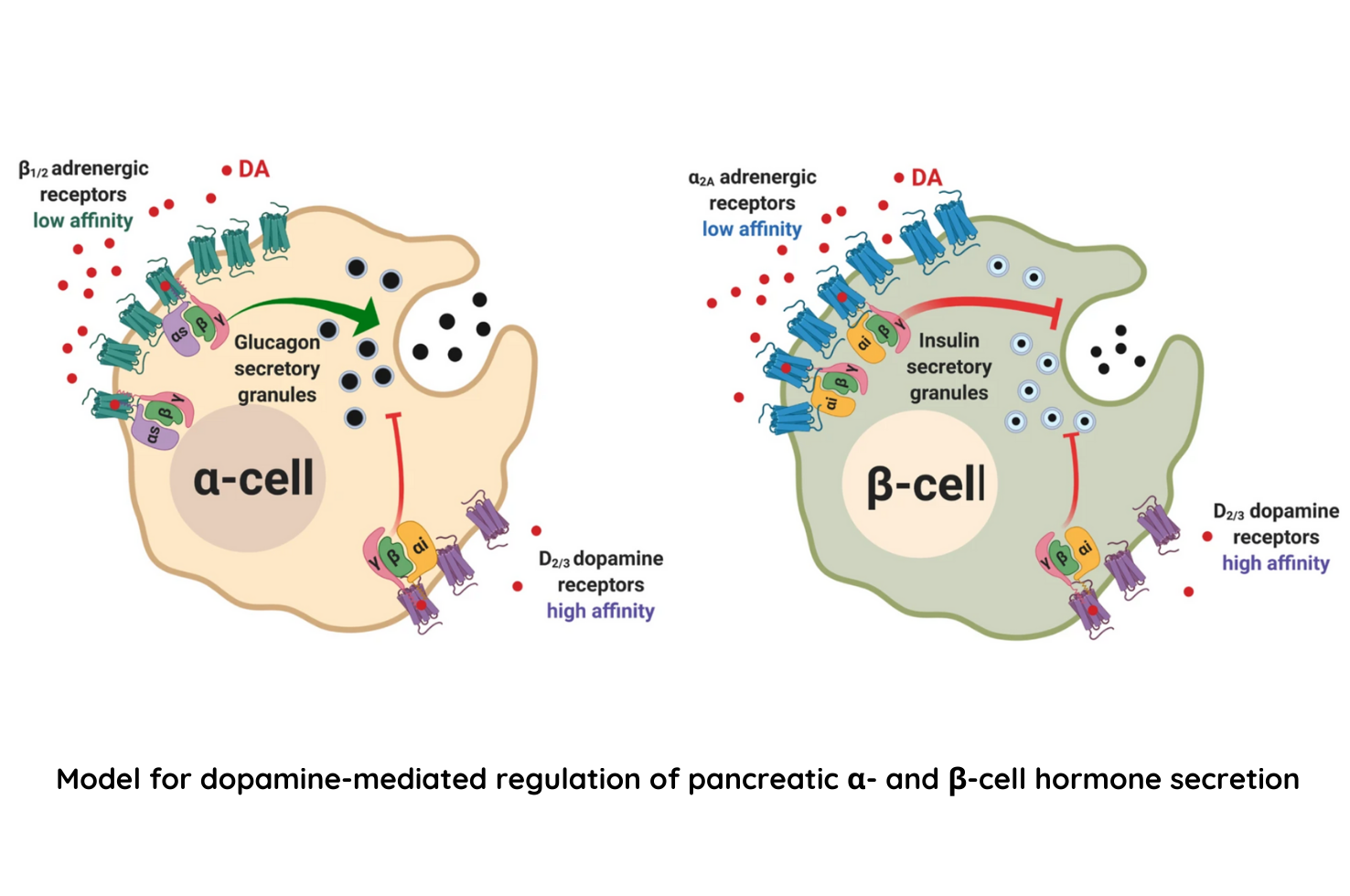
Results from the experiment demonstrated that human and mouse pancreatic α- and β-cells express the catecholamine biosynthetic and signaling machinery, and that α-cells synthesize dopamine de novo. This locally produced pancreatic dopamine signals via both α- and β-cell adrenergic and dopaminergic receptors with different affinities to regulate glucagon and insulin release. The team additionally found that dopamine functions as a biased agonist at α2A-adrenergic receptors, preferentially signaling via the canonical G protein-mediated pathway. This ability of dopamine to signal differently on dopaminergic receptors versus adrenergic receptors may be critical for finetuning the regulation of blood sugar which is essential for life.
“Our work shows that dopamine signals not only in the brain, but also directly in the periphery and antipsychotic drugs disrupt this peripheral signaling, particularly in the pancreas. This may provide an important mechanism for how these medications cause insulin resistance, and eventually diabetes. These findings pave the way for new more effective treatments for mitigating or even reversing metabolic disturbances produced by antipsychotic drugs and may even lead to better medications for treating diabetes,” said Dr. Freyberg, senior author of the study.
Dopamine regulates pancreatic glucagon and insulin secretion via adrenergic and dopaminergic receptors
Aslanoglou D, Bertera S, Sánchez-Soto M, Free RB, Lee J, Zong W, Xue X, Shrestha S, Brissova M, Logan RW, Wollheim CB, Trucco M, Yechoor VK, Sibley DR, Bottino R, Freyberg Z.
Translational Psychiatry 11, 59 (2021). https://doi.org/10.1038/s41398-020-01171-z c
