Recent Research on the Association Between Aberrant Dendritic Development and Risk for Schizophrenia
Melanie Grubisha, MD, PhD (Assistant Professor of Psychiatry), investigates the molecular underpinnings of psychiatric and neurological disease, focusing on the molecular pathways responsible for both typical and aberrant dendritic development across adolescence. Two recent papers in Neurobiology of Disease describe recent findings on the association between dendritic alterations and risk for schizophrenia.
Deciphering the alteration of MAP2 interactome caused by a schizophrenia-associated phosphorylation
Dendritic length and branching determine a neuron’s receptive field, help to segment computational compartments, and contribute substantially to how the received signals are integrated and transmitted to the cell body. Impairments in cortical neurons’ dendritic length and complexity may be linked to schizophrenia pathogenesis.
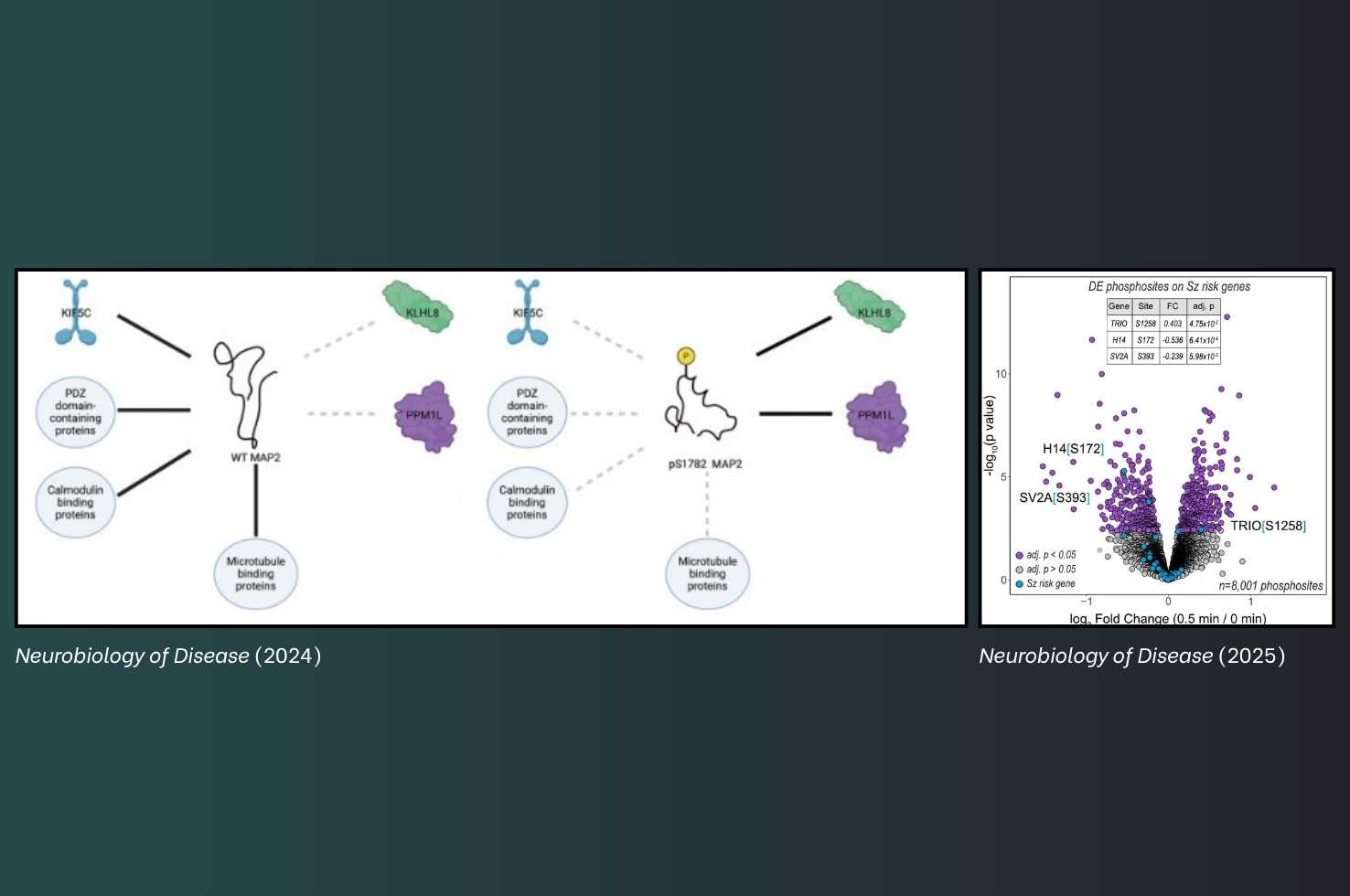
Microtubule-associated protein 2 (MAP2) is a cytoskeletal protein and a crucial regulator of dendritic structure and neuronal function, orchestrating diverse protein interactions within the microtubule network. Scientists including Dr. Grubisha investigated how hyperphosphorylation affects the MAP2 interactome, with the goal of improving our understanding of its association with the neurobiology of schizophrenia. They found that the phosphomimetic S1782E mutation in MAP2 leads to an overall loss of function in the MAP2 interactome. Reduced interactions with PDZ domain-containing proteins, calmodulin-binding proteins, ribosome proteins, and kinesin proteins may all contribute to dendritic impairments induced by S1782E, and may be linked to the pathogenesis of schizophrenia.
Lyu J, MacDonald ML, Ruiz S, Chou S, Gilardi J, Buchwald SC, Grubisha, MJ, Sweet RA
Neurobiology of Disease, Volume 203, 2024, 106731, ISSN 0969-9961, https://doi.org/10.1016/j.nbd.2024.106731
Oligodendrocyte myelin glycoprotein impairs dendritic arbors via schizophrenia risk gene Trio
During adolescence—a developmental period when the clinical symptoms of schizophrenia can emerge—the speed of cerebral cortical dendritic growth decreases, reaching apparent stability. Oligodendrocyte Myelin Glycoprotein (OMGp) expression peaks during adolescence and is associated with dendritic stabilization. However, questions remain regarding the precise signaling pathways affected by OMGp.
Dr. Grubisha and a team of scientists examined how OMGp signaling regulates dendritic architecture, and identified a point of convergence between OMGp signaling and genetic risk for schizophrenia. The investigators showed that OMGp induces differential phosphorylation of TRIO, a protein whose loss of function strongly associates with risk for schizophrenia.
Parnell E, Christiansen JM, Spratt MA, Ruiz S, MacDonald ML, Penzes P, Sweet RA, Grubisha MJ
Neurobiology of Disease, Volume 211, 2025, 106928, ISSN 0969-9961, https://doi.org/10.1016/j.nbd.2025.106928
“Schizophrenia is complex, with no single underlying cause,” said Dr. Grubisha. “Identifying points of convergence remains a rational approach for identifying new therapeutic targets. Our work aims to utilize this convergence strategy as a way to develop novel treatments that will potentially help a large proportion of patients, regardless of the heterogenous nature of upstream causes.”
